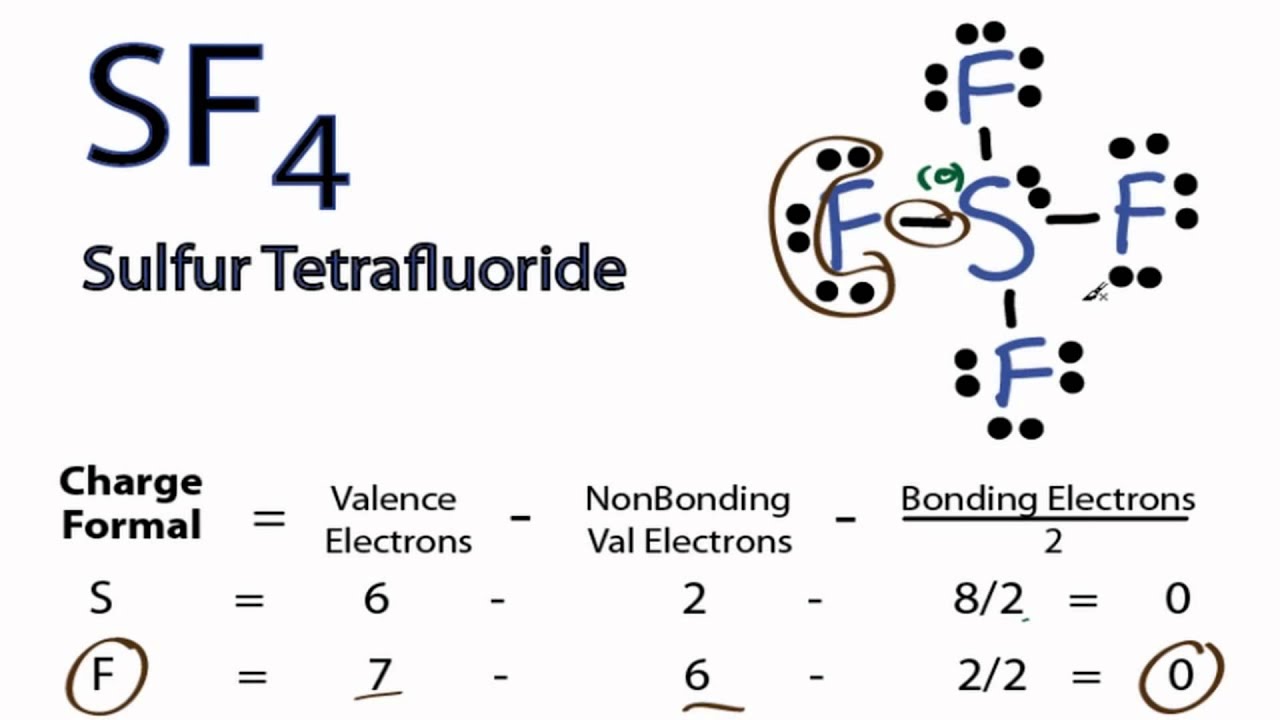
molecular shape of sf4
Solution. Steps for Writing Lewis Structures. Example 3.4.1 3.4. 1. 1. Determine the total number of valence electrons in the molecule or ion. Each H atom (group 1) has 1 valence electron, and the O atom (group 16) has 6 valence electrons, for a total of 8 valence electrons. 2.
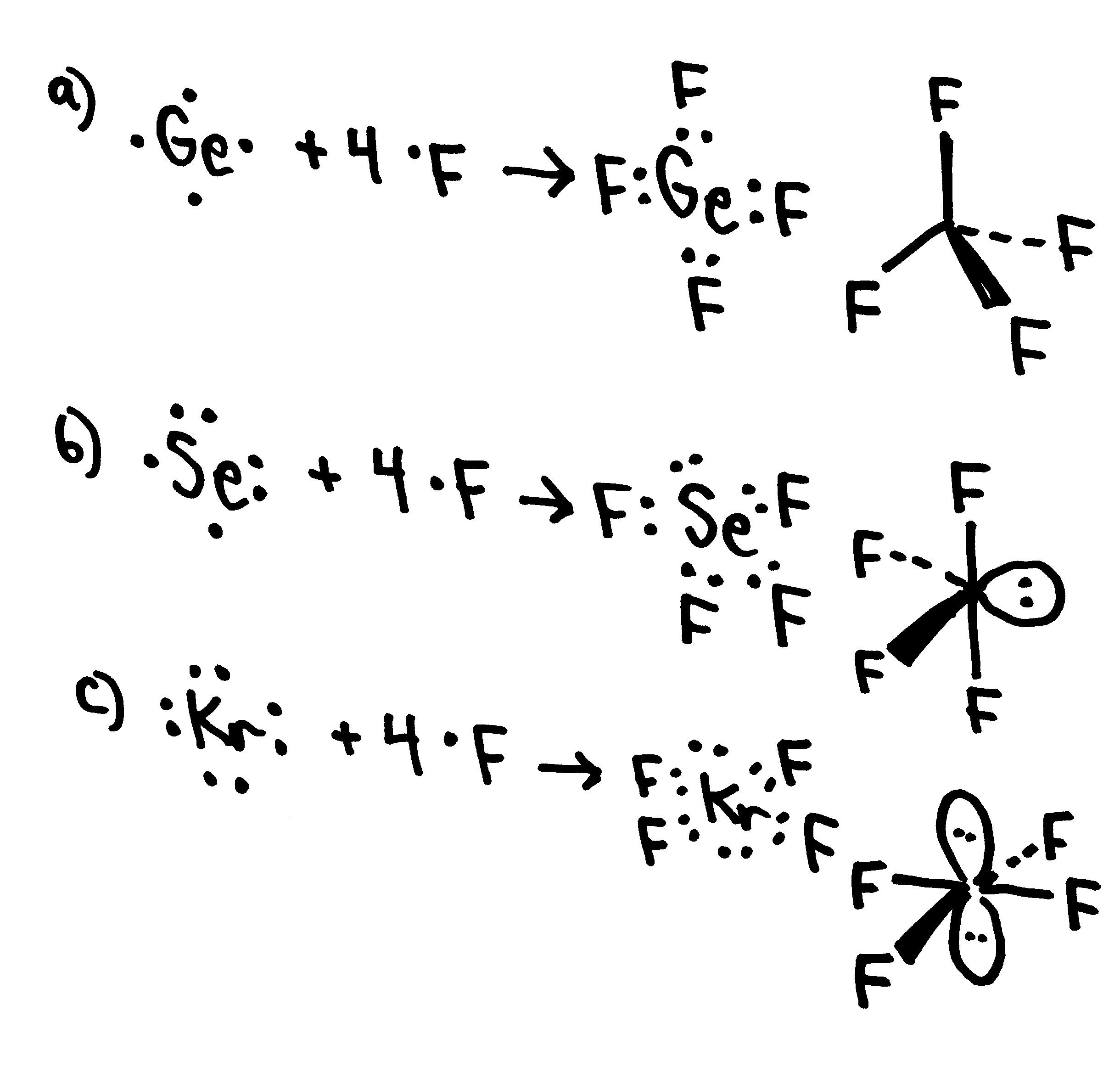
General Chemistry, chem 1b, 2nd midterm exam, spring, 20000
November 16, 2023 by Deep. The information on this page is fact-checked. Lewis structure of SeF 4. The Lewis structure of SeF4 contains four single bonds, with selenium in the center, and four fluorines on either side. There are three lone pairs on each fluorine atom, and one lone pair on the selenium atom. SeF4 Lewis Structure - How to Draw.
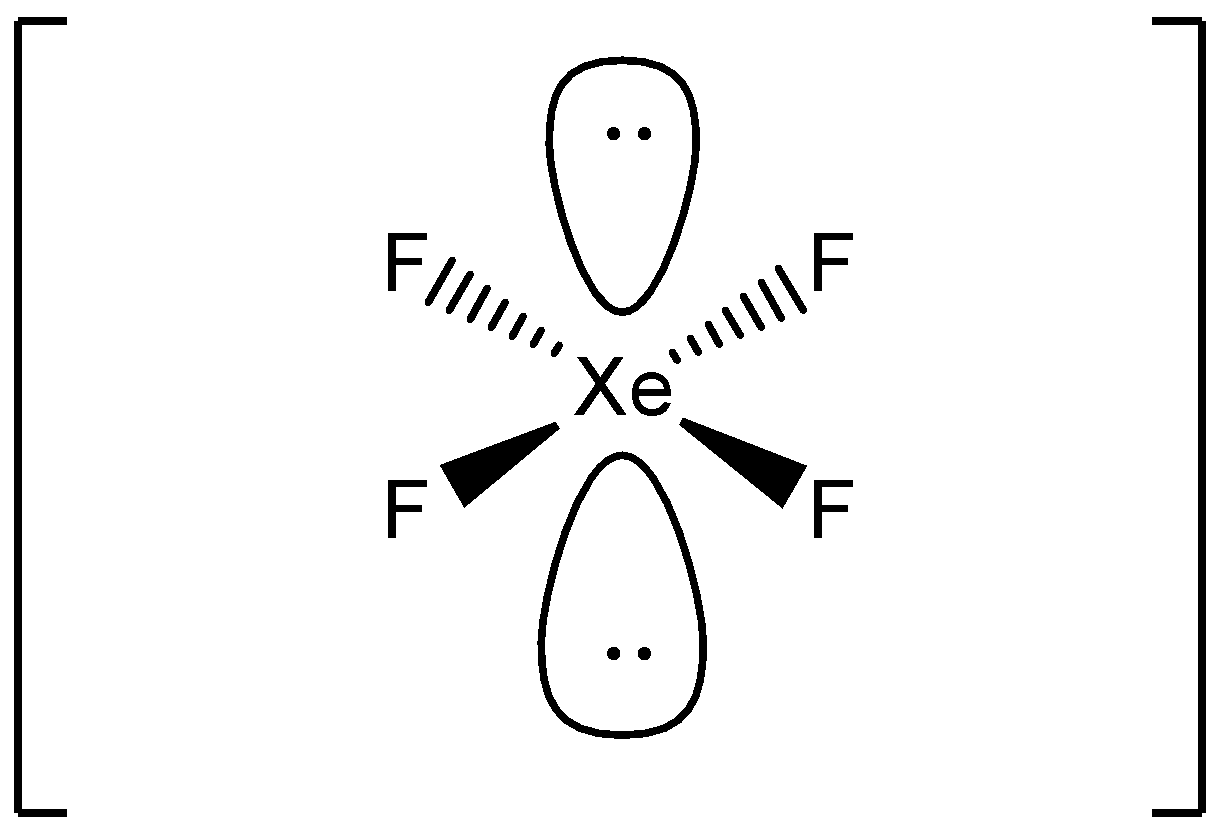
State true or false{{Xe}}{{{F}}_{{4}}} molecule is square planar in
In the SeF 4 Lewis structure, there are four single bonds around the selenium atom, with four fluorine atoms attached to it. Each fluorine atom has three lone pairs, and the selenium atom has one lone pair. Contents Steps #1 Draw a rough sketch of the structure #2 Next, indicate lone pairs on the atoms

Sf4 Polar or Nonpolar
SeF4 lewis structure is made up of one selenium and four fluorine atoms, selenium is the central atom, and fluorine is kept outside in the lewis diagram. There is one lone pair present on the central atom in the SeF4 lewis structure and 12 lone pairs on outer atoms. Follow some steps for drawing the lewis dot structure of SeF4 1.
Rncl2 Lewis Structure
An explanation of the molecular geometry for SeF4 (Selenium Tetrafluoride) including a description of the SeF4 bond angles. The electron geometry for the Sel.

Free download Lewis structure Sulfur hexafluoride Lewis pair VSEPR
A step-by-step explanation of how to draw the SeF4 Lewis Dot Structure (Selenium Tetrafluoride).For the SeF4 structure use the periodic table to find the tot.

SF4 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
Expert-verified. According to.. SeF4 Electron domain geometry: Molecular geometry: Approximate bond angles: Hybridization of central atom: sp_sp sp spåd sp?d? polarity of molecule: polar nonpolar Lewis Structure 03 Electron domain geometry: Molecular geometry: Approximate bond angles: Hybridization of central atom: sp_sp? sp sp'd spd2.

Lewis Dot Structure For Clo
- Techiescientist Is SeF4 Polar or Non-Polar? SeF4, selenium tetrafluoride, is a colorless toxic and poisonous liquid that boils at a temperature close to that of water. Se has a +4 oxidation state in this molecule. Its molecular weight is 154.96 g/mol.
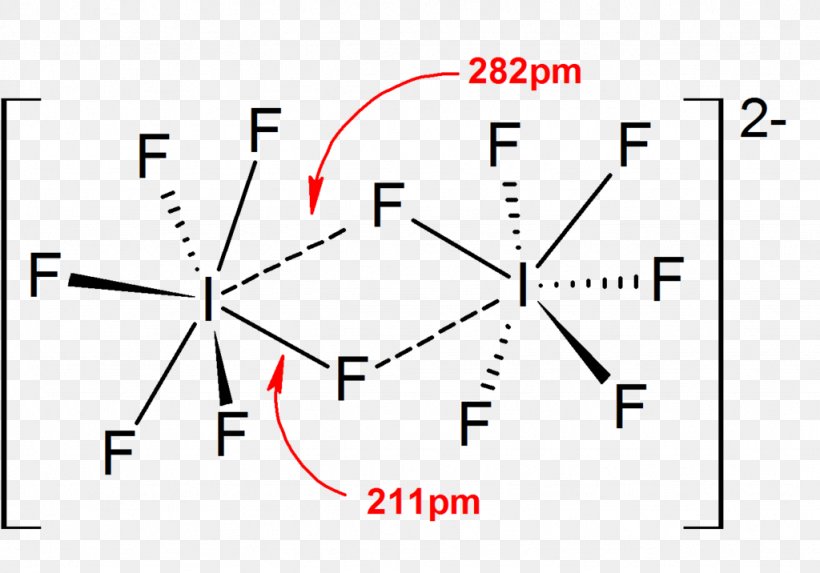
Lewis Structure Iodine Heptafluoride Triiodide Lewis Acids And Bases
Structure and bonding Selenium in SeF 4 has an oxidation state of +4. Its shape in the gaseous phase is similar to that of SF 4, having a see-saw shape. VSEPR theory predicts a pseudo-trigonal pyramidal disposition of the five electron pairs around the selenium atom. The axial Se-F bonds are 177 pm with an F-Se-F bond angle of 169.2°.
SeF4 Lewis Structure, Geometry, Hybridization, and Polarity
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
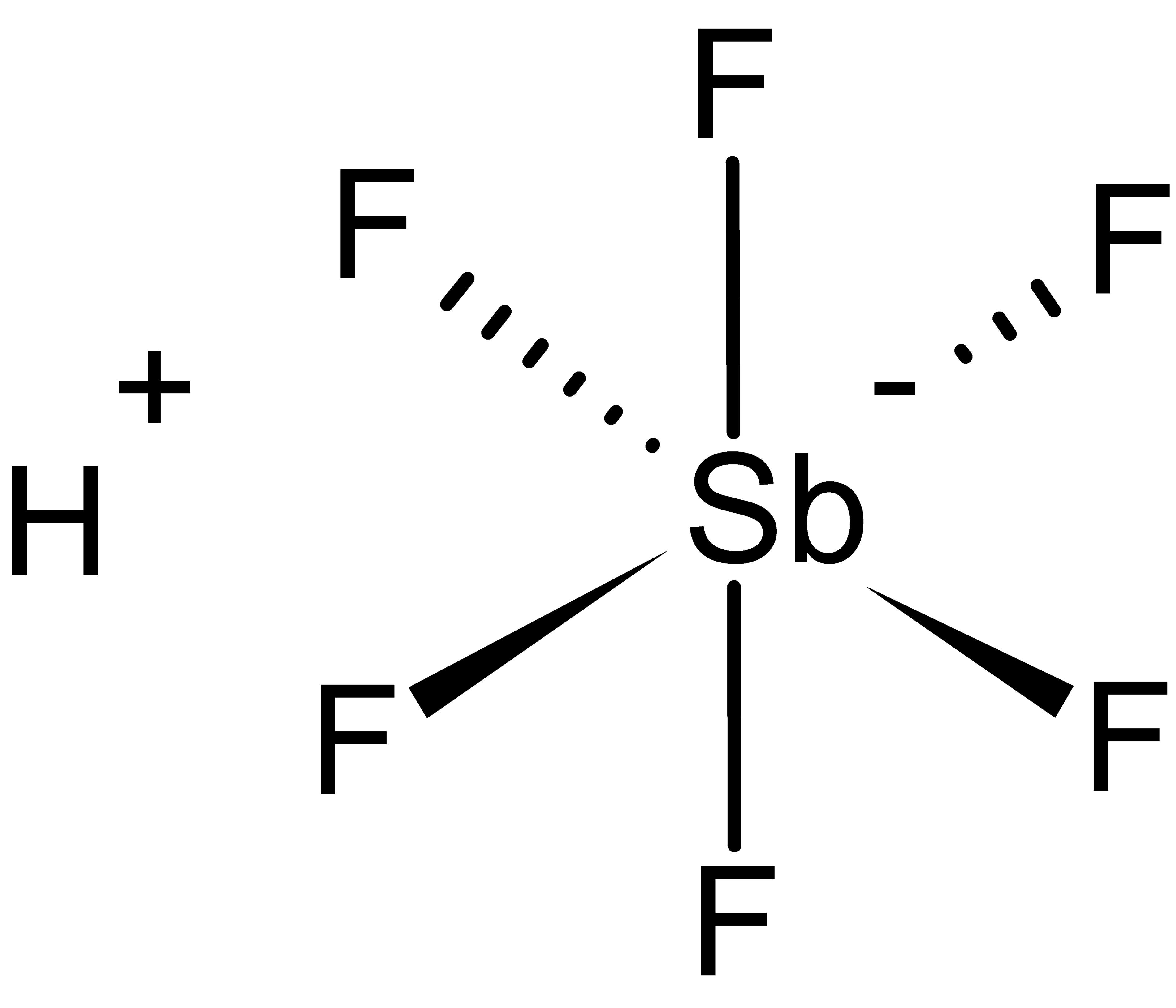
Ácido Fluorantimônico Química InfoEscola
Step 1/2. 1. SeF4: First, we need to determine the total number of valence electrons in SeF4. Selenium (Se) has 6 valence electrons, and each fluorine (F) atom has 7 valence electrons. Therefore, the total number of valence electrons in SeF4 is: 6 + 4 (7) = 34 Next, we arrange the atoms in a way that the central atom (Se) is surrounded by the.
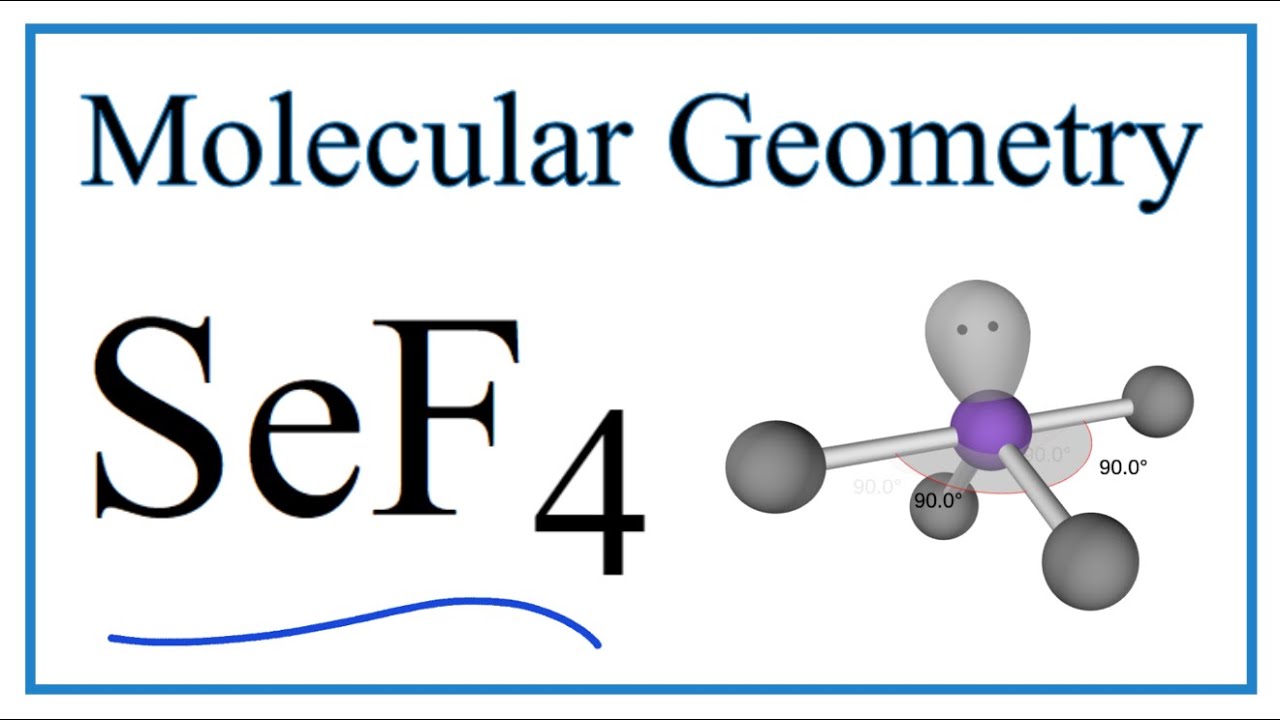
SeF4 Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
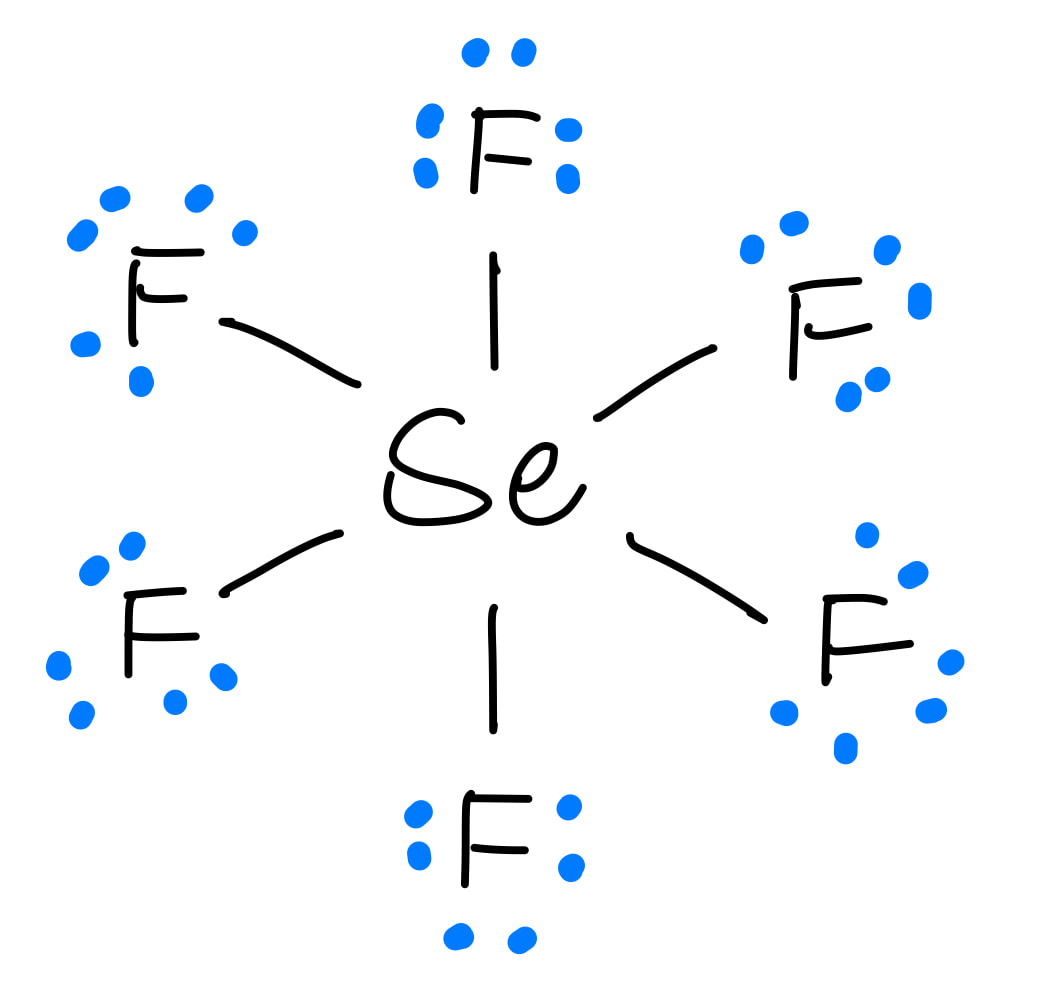
Lewis structure of SeF4 contains four single bonds between the Selenium (Se) atom and each Fluorine (F) atom. The Selenium atom (Se) is at the center and it is surrounded by 4 Fluorine atoms (F). The Selenium atom has 1 lone pair while all the four fluorine atoms have 3 lone pairs. Let's draw and understand this lewis dot structure step by step.
VSEPR
SeF4 lewis structure has a Selenium atom (Se) at the center which is surrounded by four Fluorine atoms (F). There are 4 single bonds between the Selenium atom (Se) and each Fluorine atom (F). There is 1 lone pair on the Selenium atom (Se) and 3 lone pairs on all the four Fluorine atoms (F).

Is Selenium tetafluoride (SeF4 )Polar or NonPolar? YouTube
The Lewis structure for SeF4 is written as: It can be seen in the structure that the octet for all the five atoms bonded to form the SeF4 molecule is satisfied i.e. the electronic configuration of all the four fluorine atoms as well as the selenium atom is eight. Steps of Drawing Lewis Structure of SeF4

SeF4 Lewis Structure How to Draw the Lewis Structure for SeF4 YouTube
Step 1: Count the total number of valence shell electrons on the compound Before drawing the structure, we need to know the number of valence shell electrons on all constituent atoms and their sum. Step 2: Draw the lewis dot structure for elements.

Lewis Structure Sof4
Learn to determine if SeF4 (Selenium tetafluoride) is polar or non-polar based on the Lewis Structure and the molecular geometry (shape).We start with the Le.